At ImmunityBio, we envision a day when we no longer fear cancer, but are able to conquer it, thanks to the biological wonder that is the human immune system. Our scientists are working to develop remarkable new therapies that harness that inherent power by amplifying both the innate and adaptive branches of the immune system, to facilitate the attack and elimination of cancerous or infected cells today while building immunological memory for tomorrow. The goal: to reprogram the patient’s immune system and treat the host rather than just the disease.
Smart Therapies for Difficult Diseases®
Our investigational immunotherapy products are tailored to each patient’s needs. We employ a “triangle offense” designed to deliver durable, complete responses in cancer and infectious diseases.

Investigational Cancer Therapies and Vaccines
ImmunityBio’s platforms address the entire immune system, harnessing the power of both innate and adaptive functions of the immune system by activation of ‘first responder’ natural killer (NK) cells, T cells, and macrophages, and establishment of immune memory by dendritic cells. We believe this comprehensive immunotherapy approach holds greater potential than CAR-T therapies or checkpoint inhibitors alone. We are also leveraging our established neoepitope prediction pipeline to predict which unique tumor-associated neoepitope peptides have the potential to trigger an effective, anti-tumor immune response when delivered to patients as a personalized vaccine. Clinical trials to assess the safety and efficacy of orchestrated use of these unique, complementary therapies and vaccine candidates are in progress across multiple tumor types.
Investigational Viral Therapies
Our recent findings from studies in HIV-infected patients show that cells infected with HIV can be exposed by our immune enhancer Anktiva to enable recognition and killing. This approach offers an opportunity for HIV patients to achieve a cure, and freedom from life-long anti-viral therapy. Tying viral infection and cancer together, we are developing a human papilloma virus (HPV) vaccine that has the potential to not only eradicate infection, but reduce the risk for HPV-associated cancers such as head/neck and cervical cancer.
ImmunityBio is continuously pursuing new immunotherapies designed to attack disease by enhancing the patient’s immune system, not weakening it.
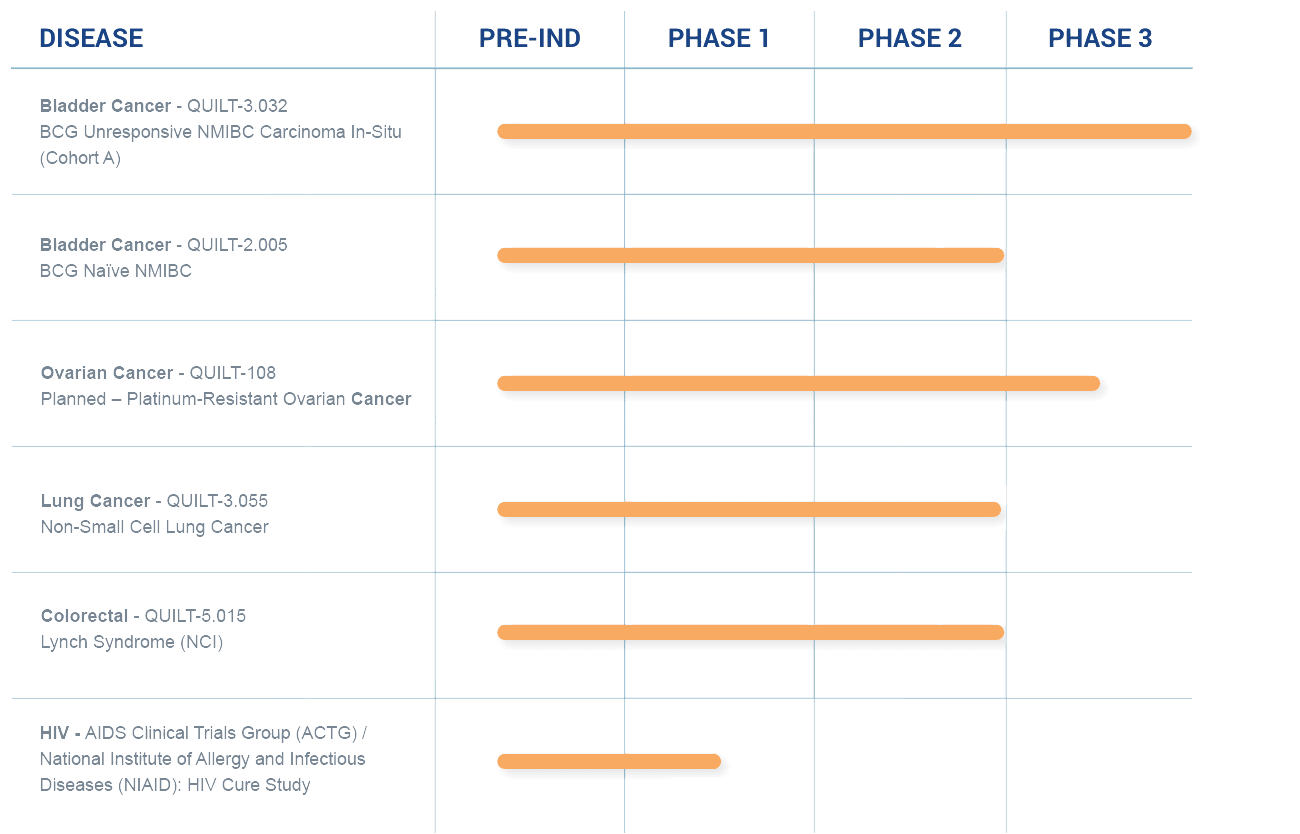
A Robust Clinical Pipeline
We are applying our science and platforms, including the development of potential cancer vaccines, to treat cancers, as well as developing immuno- and cell therapies that we believe could sharply reduce or eliminate the need for standard high-dose chemotherapy.
Latest News & Events
ImmunityBio Announces Positive Overall Survival Results of Anktiva Combined With Checkpoint Inhibitors in Non-Small Cell Lung Cancer; Meeting Scheduled with FDA to Discuss Registration Path for ANKTIVA in Lung Cancer
QUILT 3.055 trial completed and shows median overall survival almost double that of standard of care chemotherapy in...
ImmunityBio Announces FDA Approval of ANKTIVA®, First-in-Class IL-15 Receptor Agonist for BCG-Unresponsive Non-Muscle Invasive Bladder Cancer
Designated an FDA Breakthrough Therapy, the novel immunotherapy ANKTIVA activates the body’s natural killer (NK) and...
NIAID-Sponsored Study Shows N-803 Combined with Neutralizing Antibodies Could Lead to Sustained HIV Viral Control After Discontinuation of Antiretroviral Therapy
CULVER CITY, Calif., March 6, 2024 — ImmunityBio (NASDAQ: IBRX), a clinical-stage immunotherapy company, today...



