ImmunityBio is on a Mission
To continuously pursue new therapies that kill disease, not the immune system
For inquiries regarding Dr. Patrick Soon-Shiong’s recent interview on BioShield, please send us an email
A Culture Where Patients Come First
The people of ImmunityBio work tirelessly to transform our living biological technologies into innovative treatments that will improve the lives of people around the world. Our goal is to leverage and harmonize with the body’s immune system to clear disease while ensuring our therapies also protect the immune system from damage. ImmunityBio’s major focus is the development of novel treatments for advanced cancers and infectious diseases. We believe our science has the potential to change the current paradigm of care for these diseases.
We do so with a combination of highly experienced and talented people, deep scientific knowledge, and advanced research and manufacturing facilities. Together, they enable us to invent, test, and market innovative, effective, and safe therapies that address some of the greatest needs in medicine. We measure our success by the quality of life we can offer to patients.

Our History
Over the last two decades, our Executive Chairman and Global Chief Scientific and Medical Officer, Dr. Patrick Soon-Shiong, M.D., has investigated mechanisms to activate the immune system to attack tumors that can otherwise evade and escape the body’s defense mechanisms. After inventing the world’s first protein nanoparticle drug, Abraxane®, Dr. Soon-Shiong turned his focus to the next-generation of immunotherapies. ImmunityBio was founded in 2014 to create innovative immunotherapies that address serious unmet needs in oncology and infectious disease.
Since then we have progressed into a clinical-stage biotechnology company with 27 clinical trials—18 of which are in Phase 2 or 3 development—across 13 indications in liquid and solid tumors. Our proprietary natural killer cell platform can be easily expanded, genetically modified, and cryopreserved and offers multiple modes of cytotoxicity. Our lead antibody cytokine fusion protein, ANKTIVA® (N-803), has received FDA Breakthrough Therapy designation for BCG-unresponsive non-muscle invasive bladder cancer. Our work is led by a senior team with highly complementary and broad expertise across novel scientific areas including genomics and proteomics, monoclonal antibodies, fusion proteins, vaccines and autologous and allogeneic cell therapies, based on experiences at companies ranging from biotech startups to large commercial pharmaceutical enterprises.
At ImmunityBio, we never lose sight of who we serve: people with serious diseases who may be helped by the innovative therapies we conceive, design, and develop. It’s those people who inspire our team of scientists, doctors, and staff every day.
By 1990, we recognized that the key immune cell type involved in organ rejection following transplantation and in killing cancer cells was the natural killer (NK) cell – ‘nature’s first responder’. The isolation of the NK cells by Dr. Hans Klingemann in 1992 that became the NK-92 cell line, set us on our current course of developing NK cell-based therapies for the treatment of cancer and infectious disease.
How Can ImmunityBio Help You?
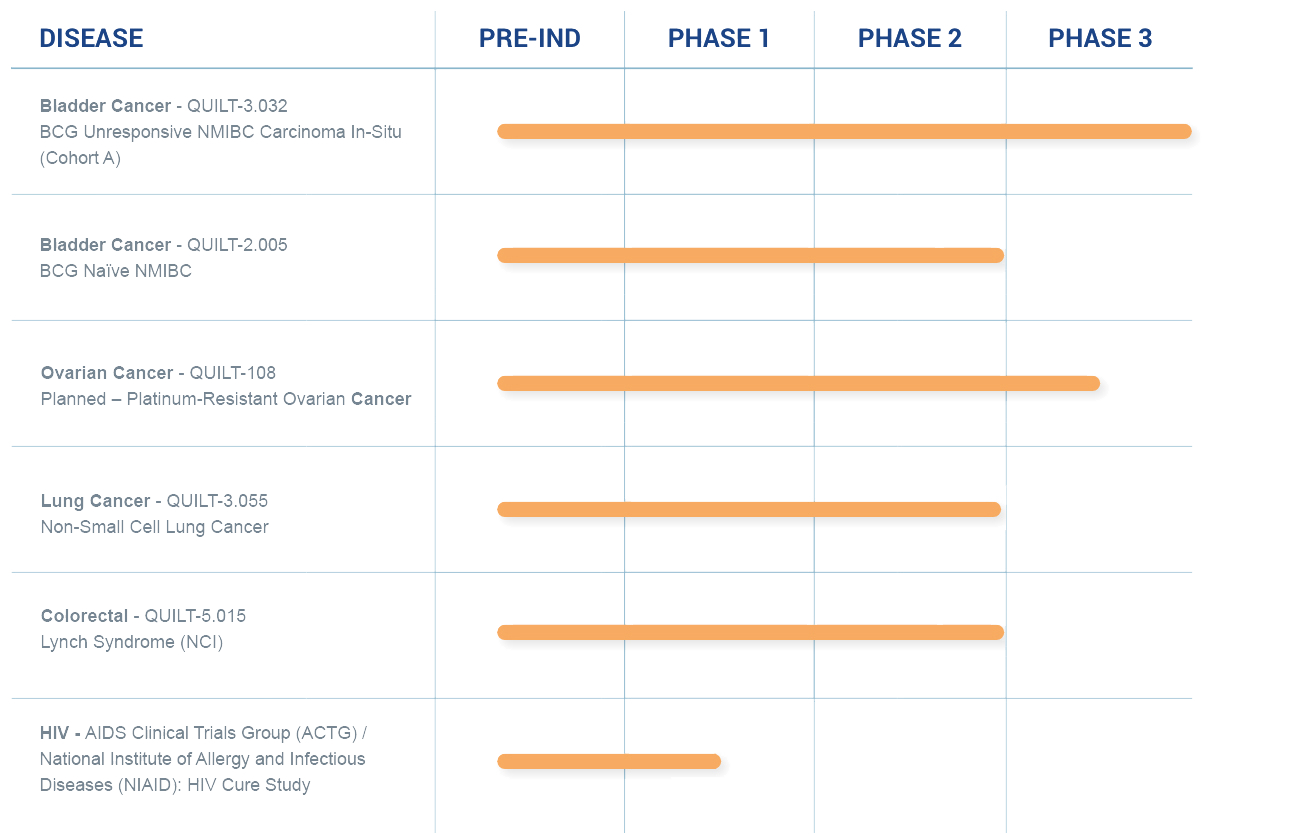
A Robust Clinical Pipeline
ImmunityBio’s immunotherapies for treating cancer and infectious diseases are currently being studied in a range of clinical trials at various stages.
Meet the Team




Liquid Tumors & Cell Therapy











Board of Directors











