Opportunities for Clinical Trial Investigators
Our investigational NK-92®-engineered cells, superagonist ANKTIVA®, and human adenovirus platform have demonstrated wide therapeutic potential across multiple tumor types and have proven to be well-tolerated, making them ideal candidates for clinical trial investigations in cancer.
If you are a current clinical trial investigator, please contact us for information.
Advance Your Medical Research
NK cell-based immunotherapies are key to next-generation cancer treatments. Our NK-92®-engineered cells have demonstrated wide therapeutic potential across multiple tumor types and have preliminary safety indicating they may be well-tolerated, making them ideal for clinical trial investigations.
ImmunityBio is actively pursuing clinical studies of our NK cell therapeutics, ANKTIVA IL-15 superagonist, and hAd5 adenovirus platform across a range of oncology and infectious disease indications. Our comprehensive approach engages both innate and adaptive immunity through cellular and cellular-activating agents, thus bringing together a whole immune system response to patients’ therapeutic needs. In contrast to other immune therapeutic approaches, our therapeutic agents work together to generate long-term immune memory—with a goal of a durable response to therapy. The frozen, off-the-shelf, blood bag format of our cellular therapies is designed to make therapy simpler, more accessible, and ready to use. These cellular therapies are being manufactured at scale for clinical studies and await investigators ready to take the next step in their patients’ immunotherapy journey.
Our Off-the-Shelf, Genetically Engineered Natural Killer Cells
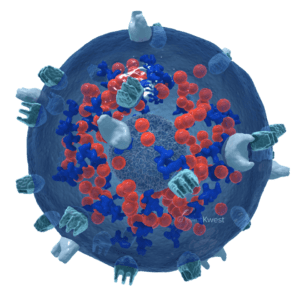
Antibody-mediated killing
haNK cells are natural killer cells engineered to incorporate a high-binding affinity receptor (CD16) that binds to an administered antibody, potentially enhancing its cancer cell-killing effect.
These antibody-targeted haNK cells directly bind to IgG1-type antibodies, such as avelumab, trastuzumab, cetuximab, and rituximab, to enhance the cancer-killing efficacy of these antibodies. They boost the available number of natural killer cells that can kill cancer cells through Antibody-Dependent Cellular Cytotoxicity (ADCC).
Antibody products are abundantly utilized to treat cancer and are estimated to generate over $100 billion in reported annual sales. A growing number of studies suggest that clinically meaningful responses to these antibody therapies correlate directly with the overall health of a patient’s natural killer cell population, and whether they express the high-affinity variant of the CD16 receptor. Current literature estimates that only 10 to 15 percent of the eligible patient population carry high-affinity CD16 receptors.1. This suggests that our haNK product candidate may have significant market potential as a combination therapy for patients who do not carry high-affinity CD16 receptors (and, as a result, exhibit a poorer response to antibody therapies).
1. Jochems, C., Hodge, J. W., Fantini, M., Fujii, R., Morillon, Y. M., 2nd, Greiner, J. W., . . . Schlom, J. (2016). An NK cell line (haNK) expressing high levels of granzyme and engineered to express the high affinity CD16 allele. Oncotarget, 7(52), 86359-86373. doi:10.18632/oncotarget.13411
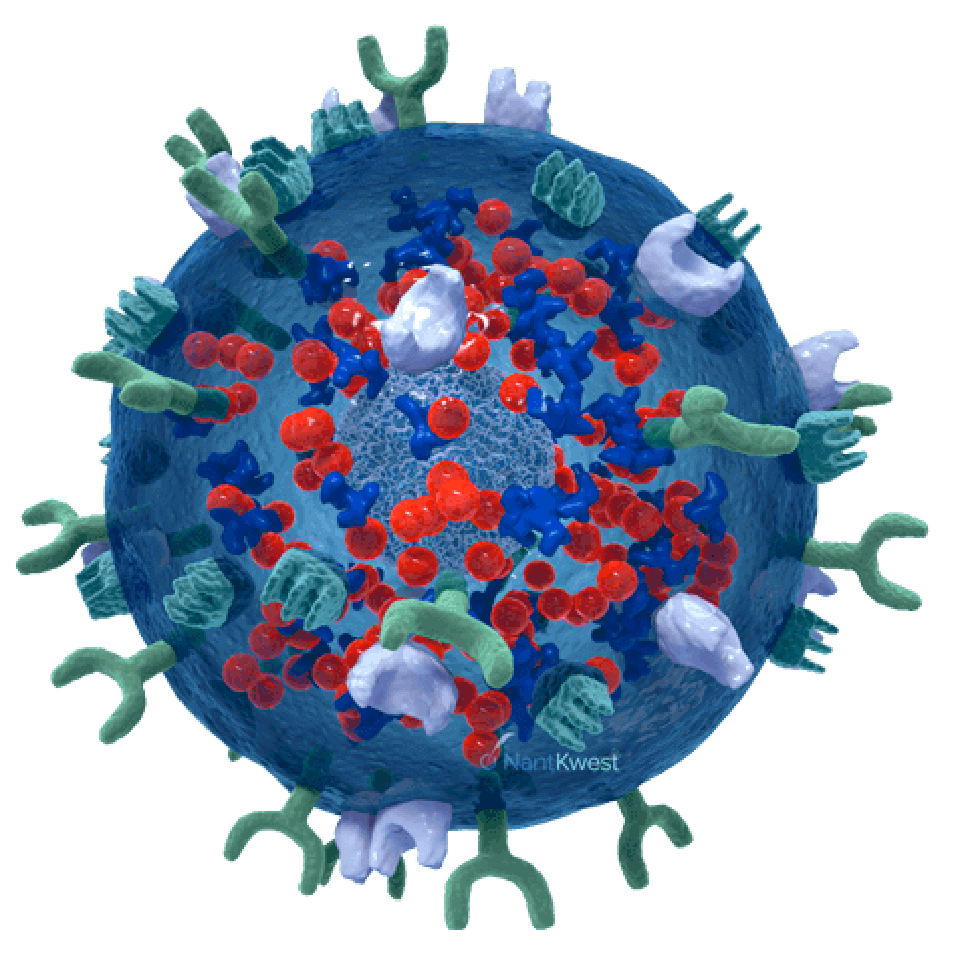
CAR-Directed and Antibody-Mediated Killing
Our newest and most promising platform for the development of therapeutic product candidates is an innovative, bioengineered combination of our haNK platform, together with a chimeric antigen receptor (CAR). The resulting product line candidates under this platform have the potential to kill in three ways: innate, antibody-mediated, and CAR-directed killing. These candidates also include one or more additional expression elements, such as functional cytokines, chemokines, and trafficking factors, making them some of the most versatile in our portfolio. These products are intended to be combined with commercially-available therapeutic antibodies to effectively target either two different epitopes of the same cancer-specific protein, or two different cancer-specific proteins.
Our novel, GMP-grade, cryopreserved, “off-the-shelf,” bi-specific, PD-L1 t-haNK natural killer cell therapy candidate targets PD-L1 via a chimeric antigen receptor (CAR), and incorporates a high-binding affinity receptor (CD16) that binds to an administered antibody, potentially enhancing its cancer cell-killing effect.
PD-L1 t-haNK is not intended for use as a monotherapy; it will be the backbone of a combination regimen that includes a therapeutic monoclonal antibody, in addition to the IL-15 superagonist Anktiva (N-803), through our exclusive co-development agreement with ImmunityBio, Inc. We believe that the addition of this selective IL-15 cytokine therapy will complement the activity of our bispecific natural killer cell therapy through its stimulation of the patient’s own resident population of natural killer and cytotoxic CD8 T-cells.
Cytokine-Enriched Natural Killer Cell Platform
Cytokine-induced memory-like natural killer cells (ceNK) are a unique set of immune cells that differentiate after a brief pre-activation with activating cytokines, and exhibit enhanced responses to cytokine re-stimulation. These cells have been isolated, and are characterized by their unique cell-surface marker profile and their highly desirable immune-memory feature. ceNK cells have pronounced immunomodulatory activity with potential applications for cancer and infectious diseases, making these cells a research focus for more than a decade. Our cytokine-enriched natural killer cell program is based on the ability to enrich and expand donor-sourced natural killer (NK) cells in a GMP facility to a clinically relevant scale. This allows for the production of a pure cytokine- activated and expanded NK cell population that possesses the unique phenotype we call ceNK cells. ImmunityBio has developed a unique portfolio of distinct ceNK cell products through the application of ImmunityBio’s proprietary bioreactors (GMP-in-a-box), cytokines, and our proprietary methods.
Investigational Therapies for Difficult Diseases
Latest News & Events
ImmunityBio is continuously pursuing new immunotherapies designed to attack disease by enhancing the patient’s immune system, not weakening it.
Our Partners