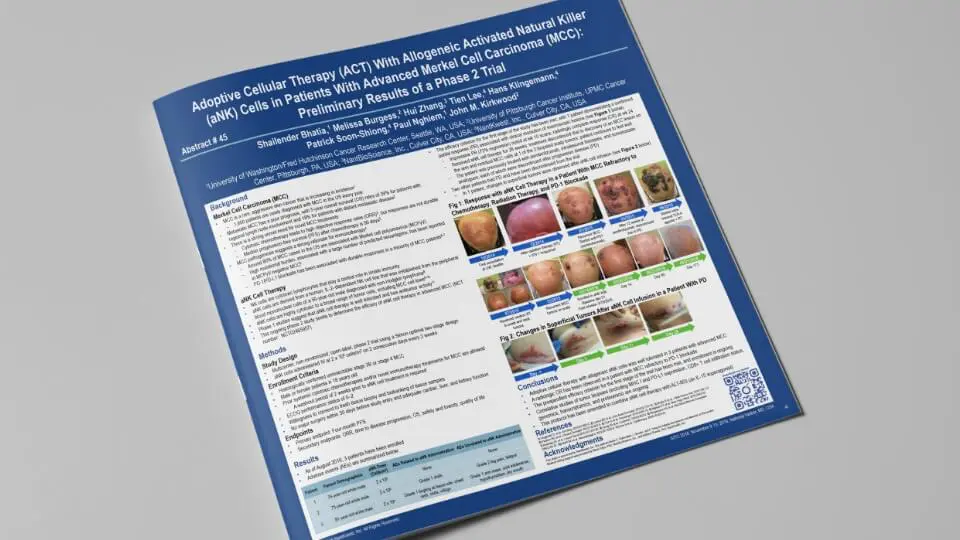
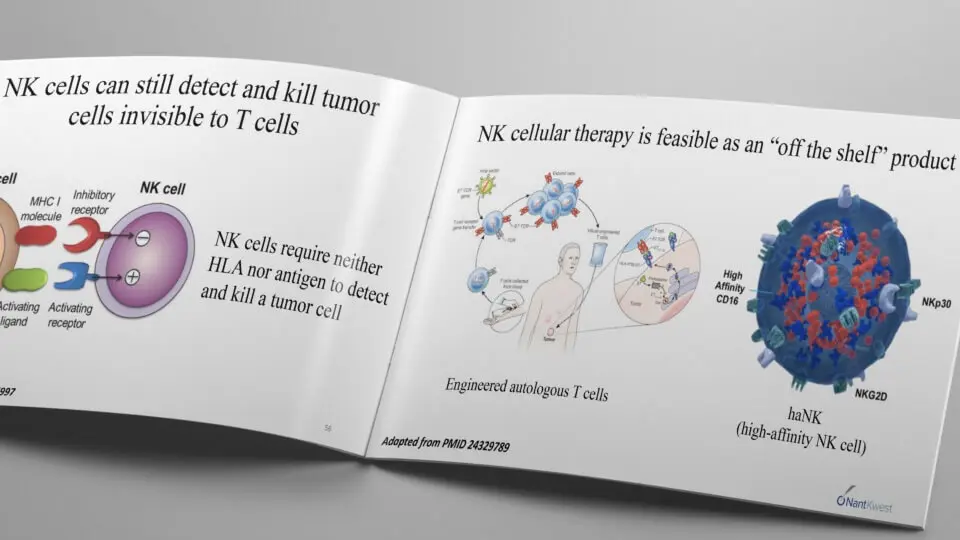
Colon Cancer
Pancreatic cancer kills an estimated 47,000 people annually; it is the fourth leading cause of cancer-related death in the U.S., and 57,600 new cases are expected in 2020.1. Less than five percent of these patients will live for more than five years after diagnosis, and the median survival prognosis is five to eight months.2
Colon Cancer Clinical Trial
Combination Immunotherapy Plus Standard-of-Care Chemotherapy Versus Standard-of-Care Chemotherapy for First-and-Second Line Treatment of Locally Advanced or Metastatic Pancreatic Cancer.
This is a phase 2, three-cohort (2 randomized and 1 single-arm), open-label study to evaluate the comparative efficacy and overall safety of standard-of-care chemotherapy versus standard-of-care chemotherapy in combination with Aldoxorubicin HCl, N-803, and PD-L1 t-haNK in subjects with locally advanced or metastatic pancreatic cancer. Each treatment setting (ie, first line maintenance, second line, or third line or greater) will be evaluated independently as a separate cohort. (NCT04390399)
Combination Immunotherapy Plus Standard-of-Care Chemotherapy Versus Standard-of-Care Chemotherapy for First and Second Line Treatment of Locally Advanced or Metastatic Pancreatic Cancer
Join a Trial Start a Trial
1. Siegel RL, Miller KD, and Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30.
2. Werner J, Combs SE, Springfeld C, et al. Advanced-stage pancreatic cancer: therapy options.
Nat Rev Clin Oncol. 2013;10:323-33.
This unique approach to orchestrating the innate and adaptive immune systems to induce immunogenic cell death may be an important new approach for pancreatic cancer patients—these being among the most challenging to treat with poor prognosis.”
Featured Research