Investigational Therapies
for Patients
ImmunityBio has developed investigational immunotherapy products that are designed to help strengthen each patient’s natural immune system, potentially enabling it to outsmart the disease and eliminate rogue or infected cells.
Harness Nature’s First Responders™
The immune system is a biological wonder that has evolved to effectively fight many infections and diseases. Thanks to advanced research, scientists are creating new therapies that harness this natural system and enhance it to fight cancers and other diseases that can outwit the immune system. At ImmunityBio, we have developed an orchestrated “triangle offense” approach that takes advantage of the natural immune system’s power to attack and kill cancerous or virus-infected cells.
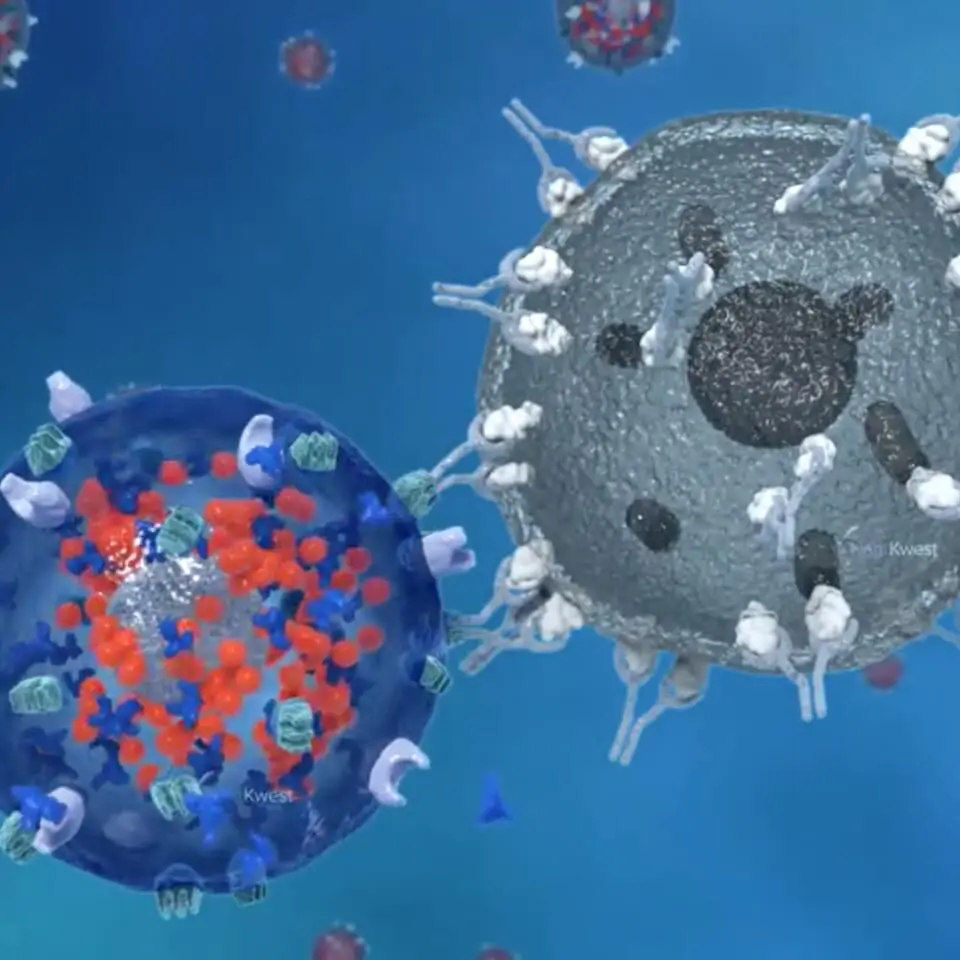
The core of our offense is our activated Natural Killer (aNK) cells. Natural NK cells, part of the innate immune system, are always on alert and ready to defend our bodies from many kinds of infection or rogue cells, such as those that cause cancer. The second aspect of our triangle offense relies on the adaptive immune system, which includes specific white blood cells called ‘T cells’. T cells engage in a deeper, prolonged attack on infected or rogue cells. T cells are a kind of reserve player that have to be activated by the innate immune system. The third piece of our strategy employs chimeric antigen receptors (CARs), which are precision attackers that seek out specific and unique markers (antigens) that exist on the surface of cancer cells.
Our unique investigational PD-L1 t-haNK™ platform combines all three of these attack modes in a single cell that can be administered by a physician (investigator) in an outpatient clinic. These bioengineered NK cells are designed to directly attack rogue cells, stimulate and recruit the adaptive immune system’s cells so they learn what to attack, and home in selectively on cancer cells by use of CARs. In PD-L1 t-haNK cells, the CAR targets the ‘checkpoint’ protein programmed death domain ligand-1 (PD-L1) that can disable the immune response if not blocked. This triangle offense approach is one of the most promising therapeutic approaches for cancer treatment that has emerged in recent years, especially for patients with difficult-to-treat cancers. Checkpoint-directed and CAR-T therapies when used alone target only the adaptive, and not the innate, immune system and despite the progress in immunotherapy represented by the development of checkpoint blockers, they fail to successfully treat approximately 70% of solid tumors. Our PD-L1 t-haNK cells are distinguished from other immune-based therapies by their ability to coordinate the innate and adaptive responses that build the ‘immunological memory’ needed for robust and durable responses.
The ImmunityBio Hypothesis
The activation of the innate immune system is key to the development of immunological memory.
Investigational Therapies for Difficult Diseases

We are Committed to Increasing Diversity in Clinical Trials
Latest News & Events
ImmunityBio, Saudi Arabia’s Ministry of Investment, KFSHRC, and KAIMRC Sign Strategic Memorandum of Understanding to Launch Cancer BioShieldTM Platform in the Middle East
CULVER CITY, Calif. and RIYADH, Saudi Arabia – May 27, 2025 – ImmunityBio, Inc. (NASDAQ: IBRX), a leading...
ImmunityBio Reports Doubled Net Revenue and 150% Unit Growth in Q1 2025, With Continued Strong Sales Momentum in First Quarter since J-code
For the three months ended March 31, 2025—marking the first quarter with a permanent J-code that streamlined billing...
ImmunityBio Requests an Urgent Meeting With FDA to Address the Change in the Agency’s Unambiguous Guidance on Jan 2025 to Submit a sBLA for NMIBC BCG Unresponsive Papillary Disease, Following an Inconsistent Refusal to File Letter on May 2, 2025
CULVER CITY, Calif., May 5, 2025 – ImmunityBio, Inc. (NASDAQ: IBRX), a leading immunotherapy company, today announced...