Founder’s Vision for
Next-Generation Immunotherapy
A Message from Patrick Soon-Shiong, M.D., Founder of ImmunityBio, Inc.
Founder’s Vision
My 35-Year Quest to Change the Paradigm of Cancer Care. Download
PDF
Founder’s Hypothesis
For the past 35 years, through my experience at UCLA treating both diabetic patients with pancreas transplants, and pancreatic cancer patients with pancreatic surgery (Whipple’s), I realized that the body had opposing immune systems: on the one hand, cytotoxic systems to reject the transplanted pancreas and on the other hand immuno-evasion systems to allow pancreatic cancer to grow. By 1990, I came to realize that the common denominator was the NK cell which on the one hand interacted with killer T cells to reject a transplanted organ and on the other hand, the killer NK and T cells were blocked by the tumor, allowing the tumor cells to grow.
At that time, I realized that the very therapy patients were receiving (high-dose chemotherapy and radiation) aided in the destruction of the very cells patients needed to cure cancer. Of concern was the realization that the standards-of-care physicians were pursuing were based on the assumption that killing the tumor and achieving a short-term response would win the war against cancer.
In fact, a short-term battle would be won by observing a response rate, but the war lost because of the destruction of important cells called lymphocytes by our chemotherapy and radiation treatment, preventing the formation of memory T cells and long-term duration of response – duration matters, T cell memory matters. Thus began the hypothesis that if we could harness the activity of lymphocytes – specifically NK and killer T cells, the potential existed to outsmart the immuno-evasion of cancer and unleash the body’s immune system to treat cancer with immunotherapy for long-term overall survival and indeed even prevent cancer in patients with high risk.
However, I faced the challenge of overcoming 50 years of standards of care which relied on high-dose chemotherapy and radiotherapy. The notion that the standard-of-care would rapidly destroy these lymphocytes, the very cells needed to kill the tumor and establish T and NK cell memory, was difficult to convince the medical establishment and regulatory authorities. The possibility that our standard-of-care was indeed responsible for preventing long-term complete remissions by the inadvertent process of destroying the very system engineered in our bodies to protect against cancer and infectious disease, was the quest that required challenging. That is the mission of ImmunityBio and the recent recognition in 2025 by the FDA that ANKTIVA and PD-L1 t-haNK was granted a Regenerative Medicine Advanced Therapy (RMAT) designation for the reversal of lymphopenia in patients receiving chemotherapy and radiation therapy is a turning point for this paradigm change in cancer care.
The destruction of the NK and T cells resulting in lymphopenia (low levels of lymphocytes) correlated with poor prognosis of overall survival across multiple tumor types. With our increasing knowledge of the immune system, including the discovery of T cells, NK cells and dendritic cells, and their role in cancer suppression (immuno-surveillance) and cancer growth when the tumor develops mechanisms to hide from the cytotoxic effects of NK and T cells (immune evasion), it became time to re-visit immunotherapy. Most are familiar with the breakthrough of CPI therapy, represented by antibodies that target either PD-1, PD-L1, or other molecules that act to inhibit immune cell (specifically T cell) responses to cancer. But even the efficacy of CPI therapies can be undermined by lymphopenia (lack of T cells), making the rescue of lymphopenia an overarching goal of my approach to cancer therapy.
This vision, which I have pursued for decades, is the transformation in the paradigm for cancer care by treating the host and activating the immune system (NK cell and killer T cell) resulting in immunogenic cell death and inducing long-term duration of response with the generation of memory T cells and memory NK cells. On the other hand, standards of care have been addressing the tumor itself (rather than the host immune system) with high-dose chemotherapy for short-term response gains.
A paradigm change would be to utilize the tumor itself in the body as an antigenic, vaccine source to generate long-term memory resulting in a durable response to the activated NK cell, T cell and memory T cell. In this way, a therapeutic cancer vaccine could be developed. Along this journey, it has been difficult to convince academia, medical cancer centers, the FDA and even the pharmaceutical industry that existing treatment paradigms for cancer are based on misplaced assumptions of administering high-dose chemotherapy and radiotherapy by “winning the battle but losing the war.” Duration of response matters and NK cells, T cells, and memory T cells are key to prolonging overall survival.
In 2017, I proposed a General Investigational Plan to study a potential therapeutic cancer vaccine applicable to all tumor types to the FDA and was given an audience with the Oncology Centers of Excellence by the FDA leadership. The hypothesis, including the request for RMAT designation presented in the plan was as follows:
A paradigm change in cancer care is required in which a modernized treatment is based on the biology of the tumor independent of anatomy, utilizing molecular and immunological insights as to the dynamic state of the cancer in its evolution (elimination, equilibrium, and escape) and specifically tailored to the patient’s cancer altered genome, to reinstate the patient into equilibrium.
The notion that the tumor tissue itself could act as a source of both antigenicity and adjuvanticity is exploited by the NANT Cancer Vaccine – a universal therapeutic cancer vaccine that acts through the orchestration of the innate and adaptive immune system across all tumor types.
We hypothesize that the normal physiological protective immune system of Elimination can be reinstated by the NANT Cancer Vaccine, first by overcoming the immunosuppressed Escape state, followed by induction of immunogenic cell death and activation of effector immune cells, with restoration of the patient to a state of Equilibrium, a paradigm change in cancer care.
The leadership at the FDA listened to this presentation and subsequently while denying RMAT designation, authorized my request to pursue this hypothesis by performing Phase 1 and Phase 2 clinical trials I termed “QUILT” QUantum Integrative Lifelong Trial across certain indications including first-line and second-line NMIBC, second-line and third-line MCC, second-line and third-line metastatic pancreatic cancer, second-line or greater GBM, third-line metastatic TNBC and third-line metastatic head & neck cancer. I also coined the term “Quantum Oncotherapeutics” to highlight the concept that the TME is dynamic and very rapid changes occur based on the therapy we administer.
For the first time in 50 years, oncologists may now have a reason to pay more attention to a complete blood count analysis of lymphocytes (NK & T cells) as the RMAT designation – granted on February 27, 2025 for ANKTIVA and PD-L1 t-haNK in combination with standard-of-care chemotherapy/radiotherapy indicated for the reversal of lymphopenia and treatment of multiply relapsed locally advanced or metastatic pancreatic cancer – is developed. Below is a summary of this journey. It is my hope that the concept of ALC will begin to be recognized by oncologists and the ratio of ANC to ALC will become a key biomarker of the current status of a patient with cancer and a predictor of outcome.
The Breakthrough of NK and T Cell Rescue by IL-15 Receptor Superagonist – Overcoming Lymphopenia
The standard analysis performed by oncologists following chemotherapy is the evaluation of the CBC with the differential subset of cells that make up the circulating blood levels. The focus of oncologists today in the review of the differential are red blood cells, platelet count and neutrophils. Remarkably, very little to no attention is given to lymphocyte count – the very cells (killer T cells and NK cells) that are critical for killing the tumor. Instead, in addition to platelet counts, oncologists today focus on the following subset of cells in the CBC:
- Red Blood Cells (RBCs) – to determine anemia as a consequence of the chemotherapy
- Neutrophils (ANC) – to determine the neutrophil level to avoid infection as a consequence of the chemotherapy
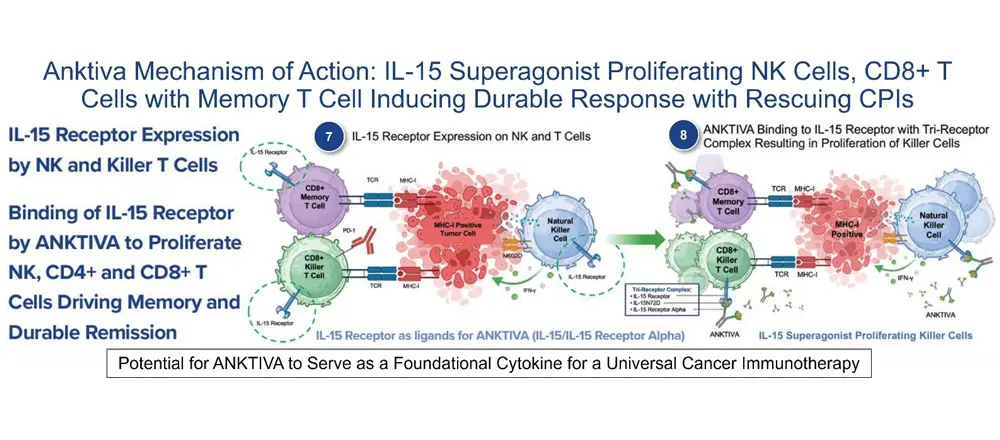
Oncologists are familiar with monitoring RBCs and neutrophils since therapies are available to mitigate the consequences of anemia and neutropenia by administration of, for example, EPOGEN to manage anemia and NEUPOGEN to manage neutropenia. Yet there is little focus on the cells of importance in the blood system that are directly involved in the killing of the tumor, lymphocytes (NK and T cells), as reflected by the lymphocyte count and percentage of lymphocytes relative to the neutrophils. It is likely that attention was rarely given to the lymphocyte count and the neutrophil to lymphocyte ratio (NLR) by caregivers managing cancer patients because no therapy existed to mitigate lymphopenia. Now, we believe our IL-15 receptor superagonist ANKTIVA (also known as N-803; molecular name nogapendekin alfa inbakicept-pmln, which has been approved for the treatment of adult patients with BCG-unresponsive NMIBC with CIS with or without papillary tumors), provides a potential treatment for lymphopenia.
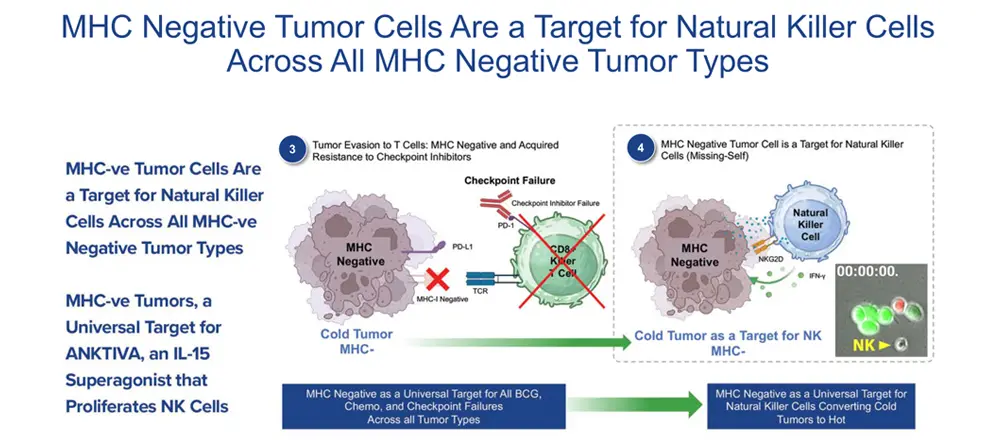
We believe that ANKTIVA may be the answer to the challenge oncologists have faced for the last 50 years, that is, how to address lymphopenia. The efficacy of immune CPIs, while a major step forward in cancer care, depends on the presence of T cells as well as the presence of the receptor to T cells on tumors (MHC-I and MHC-II). It stands to reason that with lymphopenia (low T cells) or MHC-I loss, T cell activity would be inhibited, and CPI therapy would fail. However reversing lymphopenia and overcoming low NK and T cell activity could result in the rescue of T cells and CPI failures. Just as EPOGEN and NEUOPOGEN are used across all tumor types regardless of the anatomy, we believe that NAI (proposed as ANKTIVOGEN in this potential indication), due to its ability to rescue lymphopenia by proliferation and activation of NK and T cells, has the potential to, if approved, overcome lymphopenia across all tumor types.
We believe the FDA authorization of RMAT designation for NAI and PD-L1 t-haNK in combination with standard-of-care chemotherapy/radiotherapy indicated for the reversal of lymphopenia and treatment of multiply relapsed locally advanced or metastatic pancreatic cancer places ImmunityBio on the path to a potential paradigm change in cancer care.
Lymphopenia in cancer and its effects on response rates to chemo and immunotherapy have been studied in detail. Studies performed in large patient populations with advanced cancers confirm that regardless of histological subtype and treatment, global and CD4+ lymphopenia are powerful independent predictors of risk of high-grade toxicity to chemotherapy. In addition, peripheral lymphopenia, pre-existing or induced by therapies in patients with metastatic solid tumors, strongly impacts their survival and CD4+ lymphopenia is a powerful marker of reduced survival. Different published studies exploring the impact of global lymphopenia or NK and T cell subsets on relapse-free survival and overall survival in patients with solid tumors show lymphocyte thresholds (% lymphopenia) generally <1,000 per microliter of blood leading to elevated relative risk scores for both relapse-free survival (1.35-3.81) and overall survival (1.25-7.70).
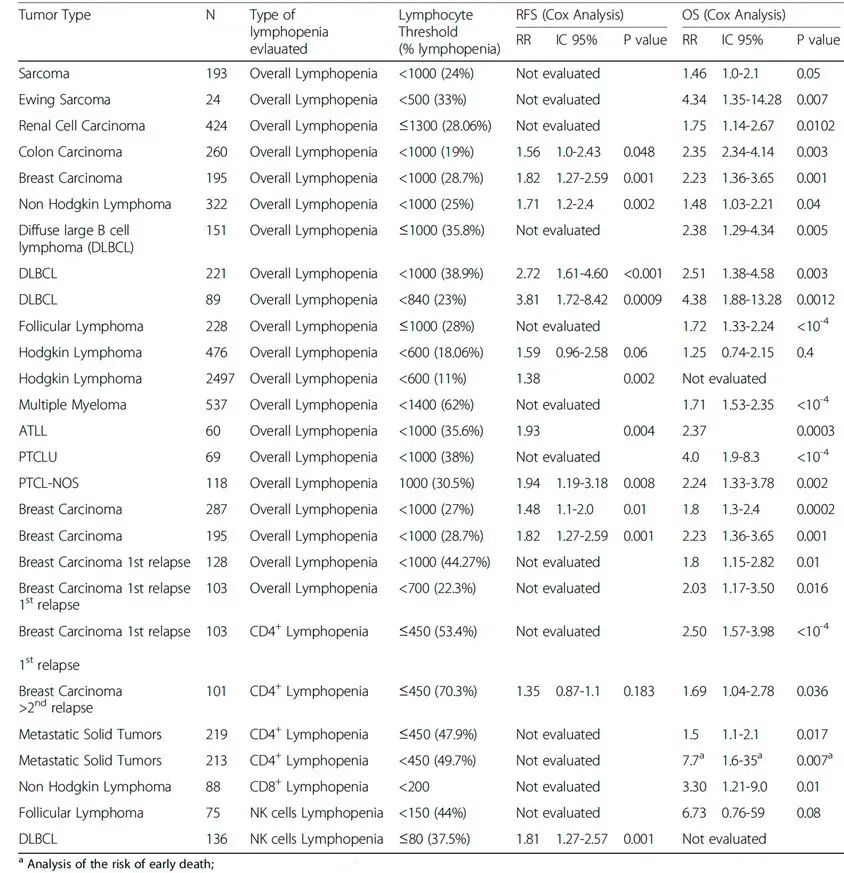
We believe the FDA approval of ANKTIVA with BCG for treatment of adult patients with BCG-unresponsive NMIBC with CIS with or without papillary tumors in 2024 and the recent RMAT designation with ANKTIVA and PD-L1 t-haNK provides the opportunity for the first FDA-approved cancer therapy (ANKTIVA in BCG Unresponsive NMIBC CIS) to be extended in patients receiving standard-of-care chemotherapy and radiotherapy, that has the potential to reverse lymphopenia – meaning the ability to resuscitate and rescue NK and T cells – the very cells of critical importance for immunogenic cell death and long-term memory against the tumor. Through its mechanisms of action to proliferate NK and T cells (as described on its label) and therefore rescue lymphopenia, I believe ANKTIVA has the potential to be the backbone of my vision for ‘Immunotherapy 2.0’ that could launch a new era and paradigm change in the treatment of cancer across all tumor types by driving memory T and NK cells with durable remission of the disease.
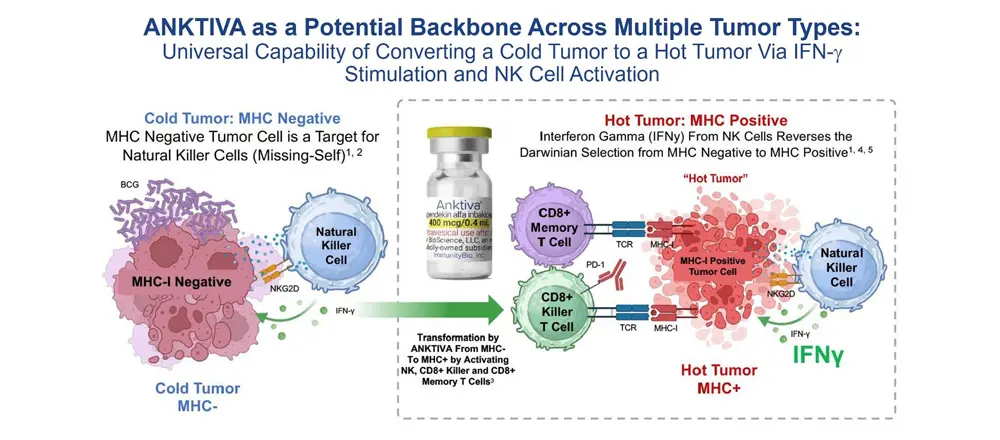
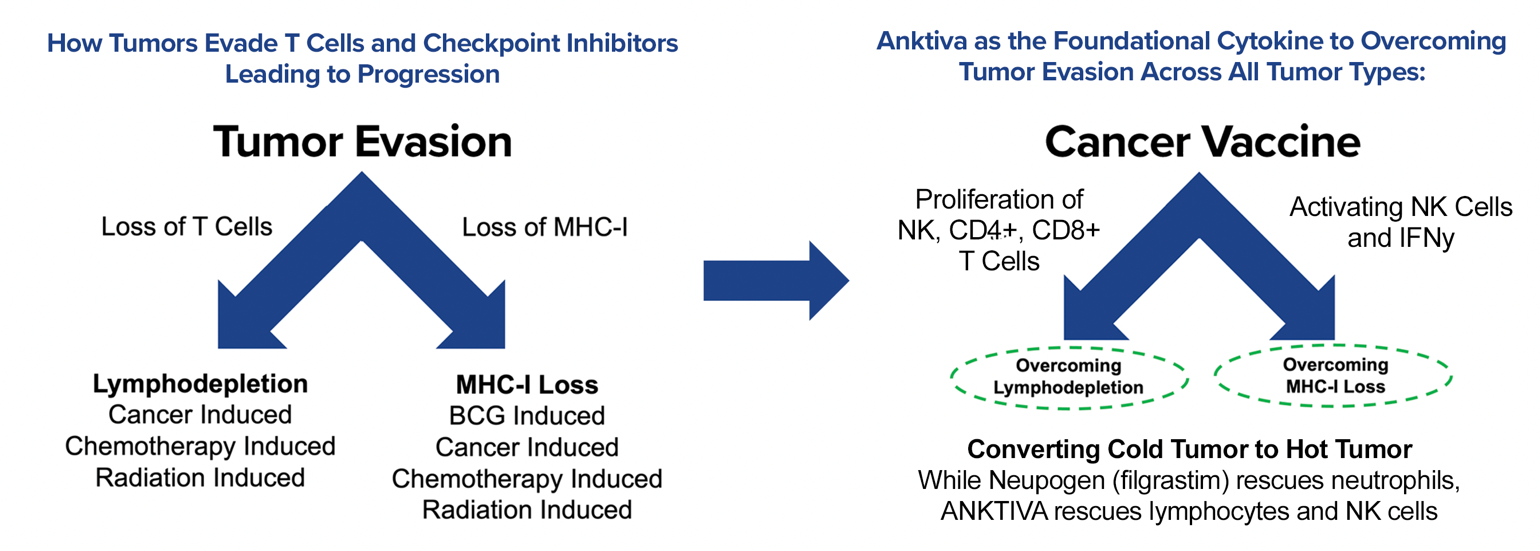
ANKTIVA overcomes tumor evasion by mitigating both the loss of T cells (lymphopenia) and the loss of the ligand to T cells on the tumor (MHC-I loss). The mechanism of potentially overcoming cold tumors will be discussed in detail further below.
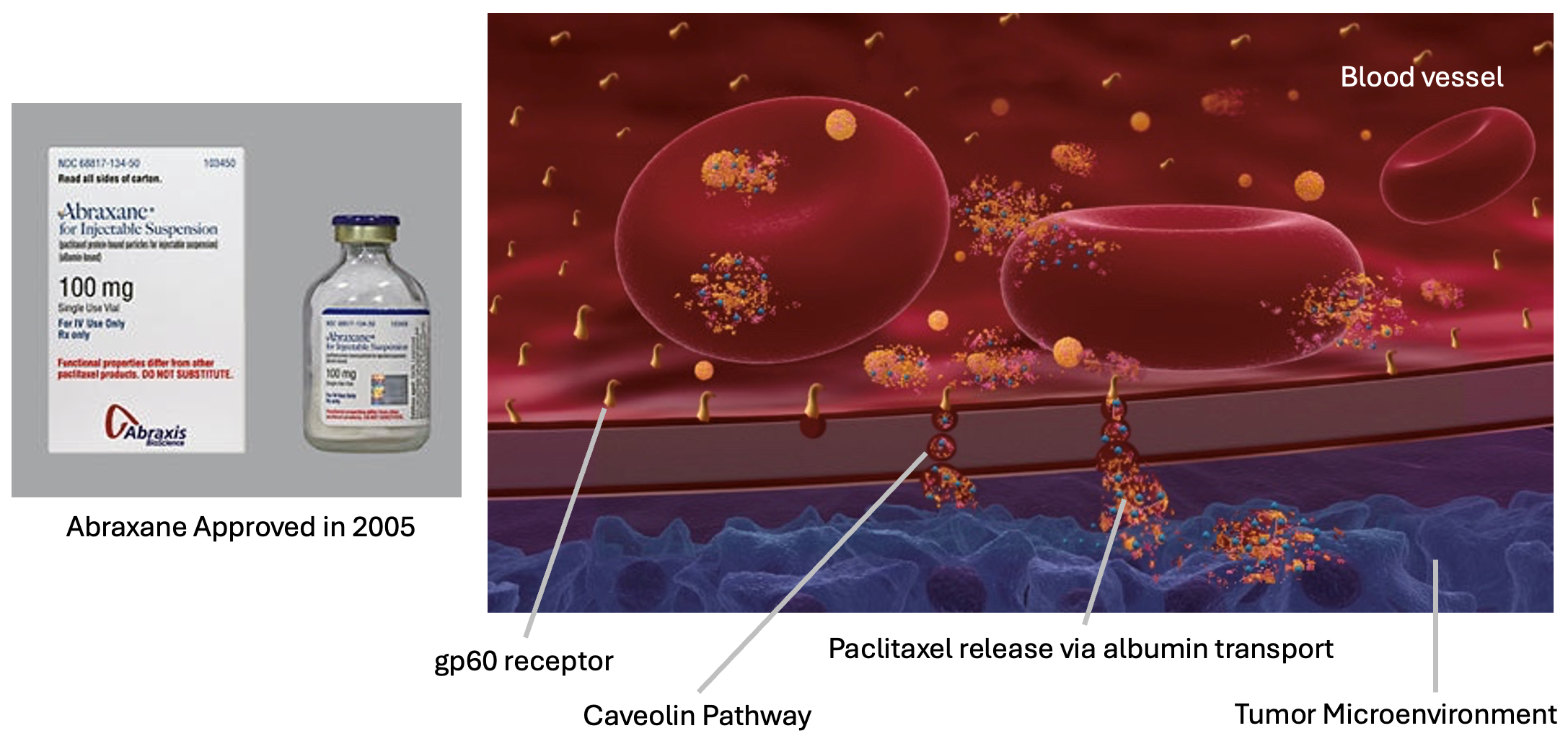
ABRAXANE – Immunogenic Cell Death in the Tumor Microenvironment (2005: FDA Approval)
As the first step in my quest to change the paradigm of cancer care previously based on toxic high-dose chemotherapy and/or radiation, I left academia (UCLA) to develop the nation’s first albumin-bound nanoparticle of paclitaxel (nab-paclitaxel), ABRAXANE, and to institute the concept of LDMC to elicit a vaccine-like effect. LDMC is chemotherapy not used at the MTD to kill the tumor, but rather to stress the tumor and elicit release of antigens to allow the killer cells surrounding the tumor (macrophages, T cells and NK cells), to induce immunogenic cell death.
Even today, the concept of albumin-bound therapy to concentrate drug at the tumor site is not always fully appreciated by the oncology community. ABRAXANE was developed to exploit the biology of the concept of transcytosis whereby the transport of albumin-bound paclitaxel across the endothelial blood vessel layer to the TME is accomplished through the gp60 receptor and the Caveolin pathway leading to entry of paclitaxel in the TME and the production of stress molecules in the tumor (DAMPs), while at the same time activating the killer macrophages (M2 suppressive macrophages to M1 killer macrophages). ABRAXANE was approved by the FDA in 2005 for the treatment of breast cancer after failure of combination chemotherapy for metastatic diseases or relapse within six months of adjuvant chemotherapy and now also has FDA approval for patients with lung and pancreatic cancer in certain indications.
ANKTIVA – IL-15 Receptor Superagonist Rescuing Lymphocytes and Overcoming MHC-I Loss (2024: FDA Approval)
With ABRAXANE as the first pillar in this quest to change the paradigm of care, I then turned my attention to addressing lymphopenia and unleashing the “triangle offense” via the IL-15 receptor superagonist ANKTIVA.
In 2024, ANKTIVA with BCG for the treatment of adult patients with BCG-unresponsive NMIBC with CIS with or without papillary tumors was approved by the FDA, who authorized the description of the mechanism of action in the label as:
Nogapendekin alfa inbakicept-pmln is an IL-15 receptor agonist. IL-15 signals through a heterotrimeric receptor that is composed of the common gamma chain (γc) subunit, the beta chain (βc) subunit, and the IL-15-specific alpha subunit, IL-15Rα. IL-15 is trans-presented by the IL-15 receptor α to the shared IL-2/IL-15 receptor (βc and γc) on the surface of CD4+ and CD8+ T cells and NK cells. Binding of nogapendekin alfa inbakicept-pmln to its receptor results in proliferation and activation of NK, CD8+, and memory T cells without proliferation of immuno-suppressive T-reg cells. In vivo, intravesicular nogapendekin alfa inbakicept-pmln alone or in combination with BCG showed anti-tumor activity when compared to BCG alone, in a carcinogen-induced model of bladder cancer in
immunocompetent rats.
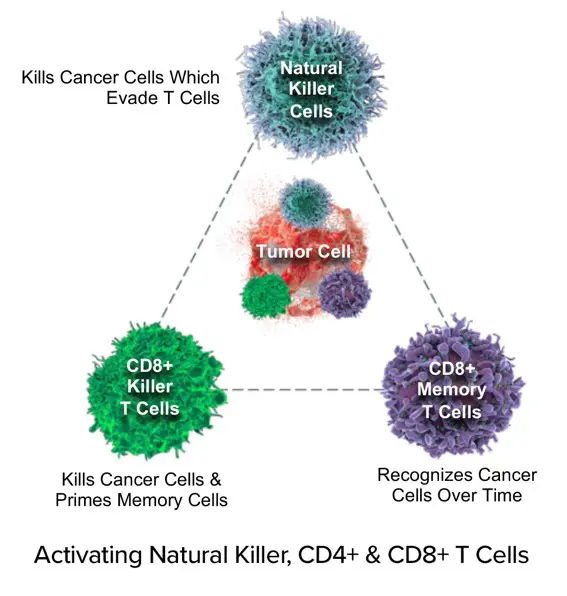
I believe this potentially universal therapeutic vaccine, sometimes referred to as NCV (U.S. Patent #US11071774-B2), could coordinate the release of antigens from tumor cells (DAMPs/neoantigens and/or shared tumor-associated antigens, TAAs) or provision of vaccine antigens with support for immune-cell activity, including cells of both the innate and adaptive immune systems, to achieve a vaccine-like effect. ANKTIVA, due to its mechanism of proliferating and activating innate (macrophages, NK cells, dendritic cells) and adaptative (T cells, iNKT cells, and memory T cells) immune cells provides the vital stimulus needed to establish immune memory.
Thus, I believe ANKTIVA has the potential to become the backbone for a cancer vaccine not only to potentially cure cancer (long-term duration free of disease) but also to prevent cancer. An example of the possibility of prevention is in subjects with Lynch Syndrome, a genetic condition that puts them at significantly higher risk for cancer, and with an earlier age of onset. Currently, ANKTIVA plus vaccines that deliver tumor associated antigens via adenovirus, relevant to Lynch Syndrome are being tested in clinical trials with subjects with Lynch Syndrome for their ability to prevent cancer onset. If efficacious, this novel treatment could help support that a universal cancer vaccine is indeed possible.
Quantum Oncotherapeutics – A Potential Universal Therapeutic Cancer Vaccine
Changing the paradigm and convincing oncologists of the need to avoid high-dose chemotherapy and implement a treatment protocol that activates the patient’s immune system rather than destroying the NK cells and T cells remains a difficult task even today.
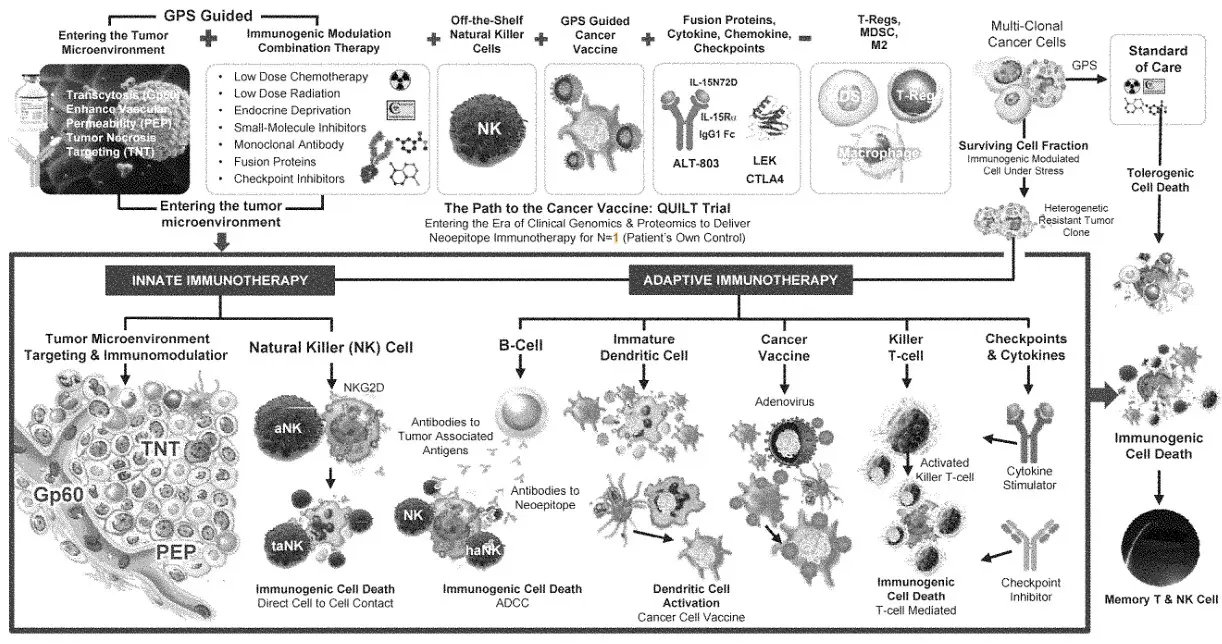
In 2017, the FDA allowed ImmunityBio (formerly NantKwest and NantCell) to initiate a series of transformative clinical trials in which the concept of inducing immunogenic cell death (ICD) was pursued. These trials entitled “QUILT” QUantum Integrative Lifelong Trials were seminal in the pursuit of a universal cancer vaccine. The clinical protocols shared an approach that followed my vision of inducing DAMPs, avoiding high-dose chemotherapy, educating T cell via dendritic cell activation and inducing NK, CD4+ and CD8+ T cell proliferation in combination with off-the-shelf CAR-NK cell therapy to overcome immunosuppressive T-reg cells and myeloid-derived suppressive cells (MDSCs). The findings from the QUILT trials have confirmed the validity of the NCV approach.
Video: 2022 Presentation of the Future Vision of a Path to a Universal Therapeutic Cancer Vaccine
In 2022, I was honored to present the concept of “Quantum Oncotherapeutics” providing updates and receiving authorization to proceed with the QUILT trials and by 2025 met with the FDA to present the update of these trials.
The Challenges of Checkpoint Failure Facing Oncologists: Immunotherapy 2.0 Beyond Checkpoints
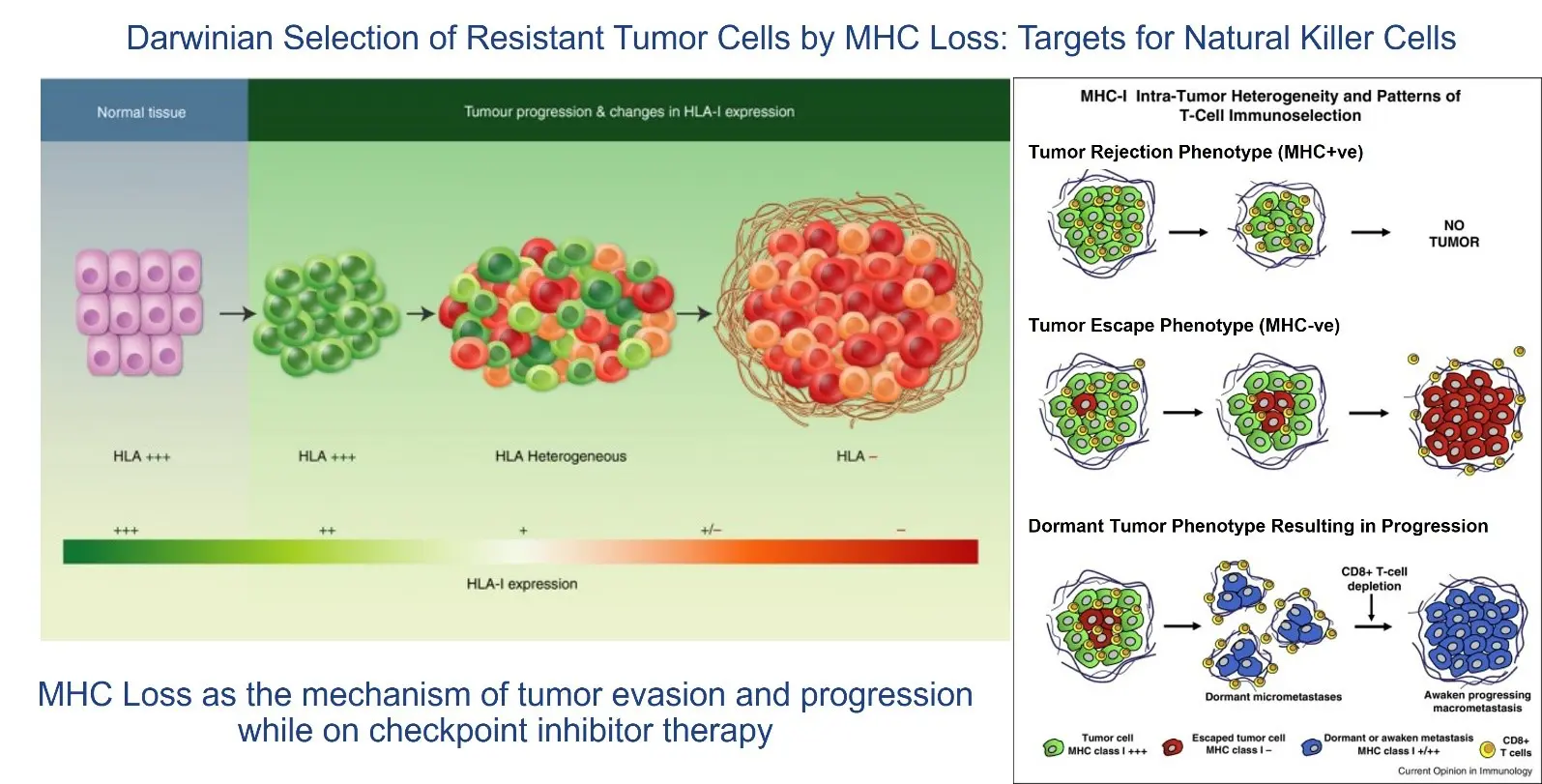
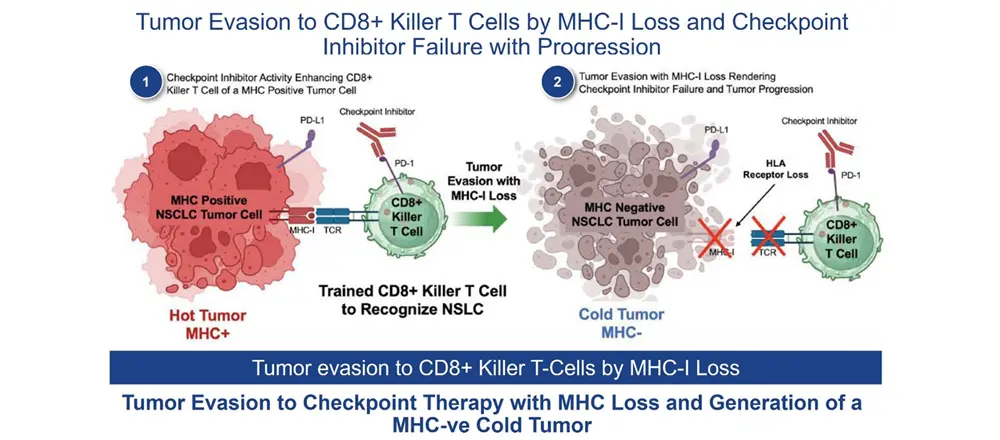
By 2024, the FDA had approved the use of CPIs (pembrolizumab, nivolumab, etc.) across 17 different tumor types. However, at the 2024 ASCO Annual Meeting I believe it became apparent that responses to CPIs were frequently fleeting and that CPIs were not effective in so-called “cold tumors.” The cold tumor is defined as lacking sufficient T-cell infiltration or activity of the T cells, even with use of a CPI, in the TME, and the loss of MHC-I, the binding site on the tumor for the T-cell receptor. Thus, the lack of effect in cold tumors is driven by fewer T cells (lymphopenia) and selection of cancer cell clones without the receptor for T cells to bind to MHC-I (MHC-I loss). Given these dynamics, it is not surprising that CPIs alone often fail or elicit only transient responses.

Overcoming Tumor Evasion and Converting a Cold Tumor to a Hot Tumor
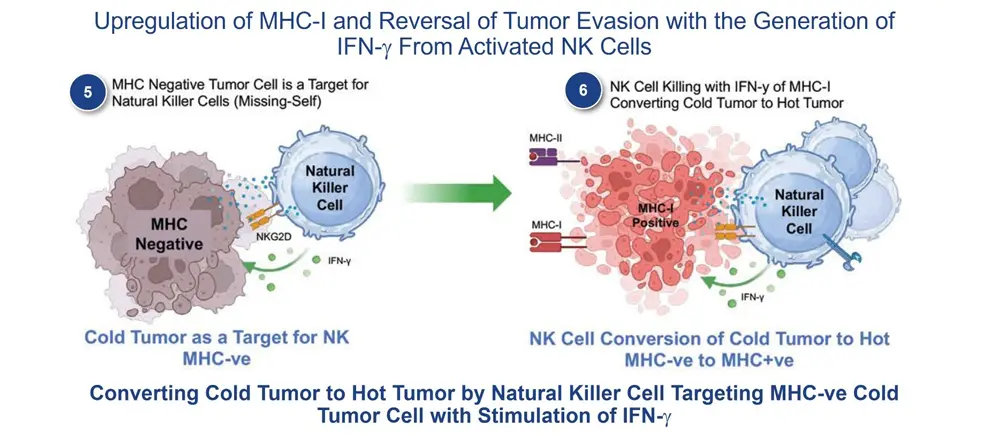
The addition of ANKTIVA not only rescues T cells, but also activates NK cells that have the ability to recognize tumor cells without MHC-I expression and, as a result of increased interferon-γ (IFN-γ) secretion by the activated NK cells, also has the potential to restore MHC-I expression. All of these activities act to rescue CPIs. Thus, the combination of ANKTIVA plus a CPI represents Immunotherapy 2.0. A depiction of how ANKTIVA has the potential to convert a cold tumor to a hot tumor and rescues CPIs is provided below.
QUILT Studies Validate NCV with ANKTIVA as the Backbone
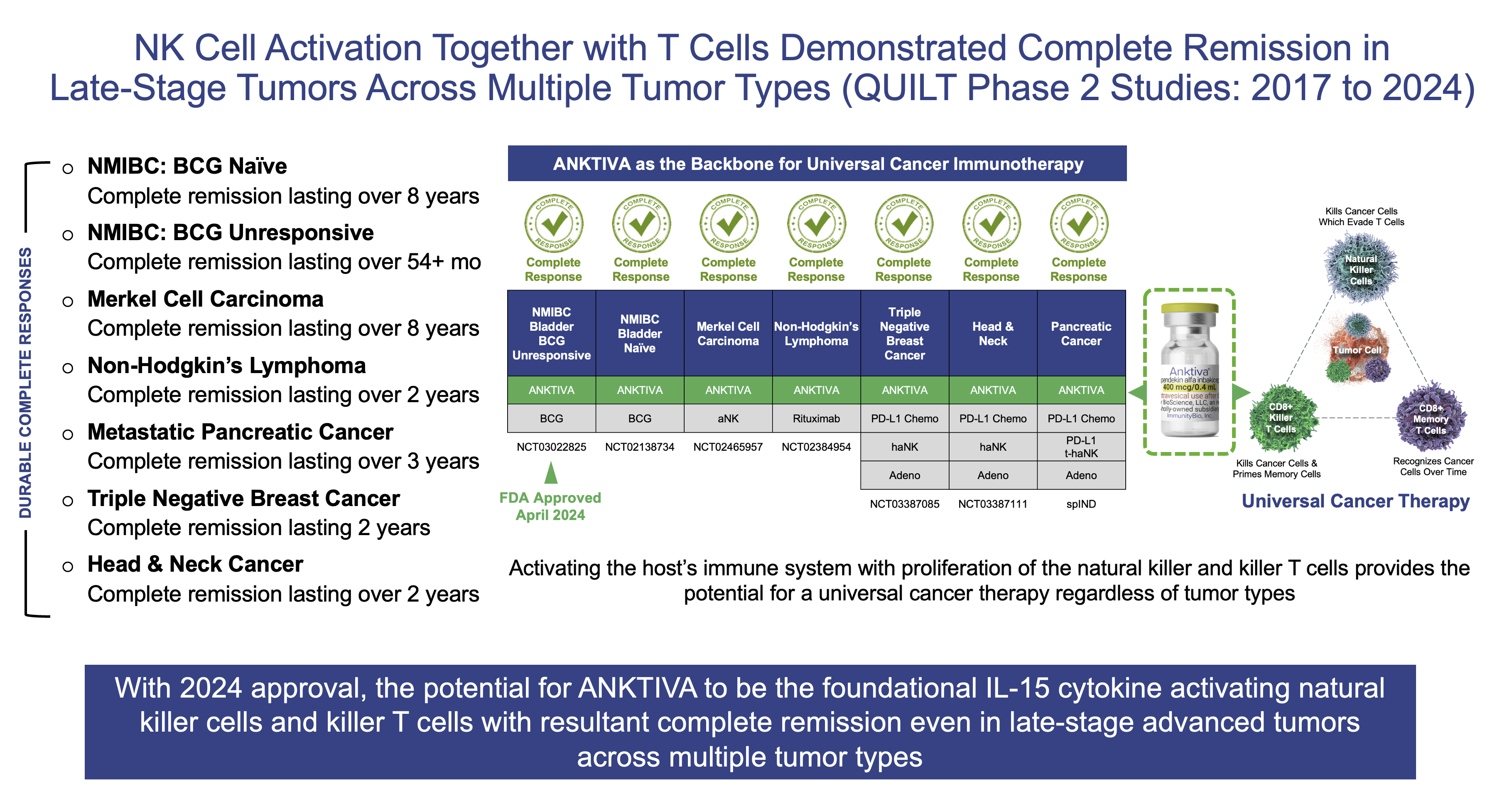
I presented findings from completed QUILT studies during a 2025 program review meeting with the FDA, including the achievement of complete remissions in late-stage and advanced tumors of many types, such as NMIBC, MCC, TNBC, pancreatic cancer and head & neck cancer, validating the concept of eliciting immunogenic cell death rather than the tolerogenic cell death induced by current standards of care. A summary of the complete remissions is shown in the image below.
ImmunityBio Platforms – The Development of a Universal Therapeutic Cancer Vaccine
Based on the vision of activating the patient’s immune system, the company continues its efforts to demonstrate that a universal cancer vaccine that leverages the power of its platforms – backbone ANKTIVA, off-the-shelf and autologous NK-cell-based therapies, adenovirus-vectored delivered vaccines, and other technologies – provide benefit and hope not only for patients with cancer but also for those with a high risk of developing cancer.
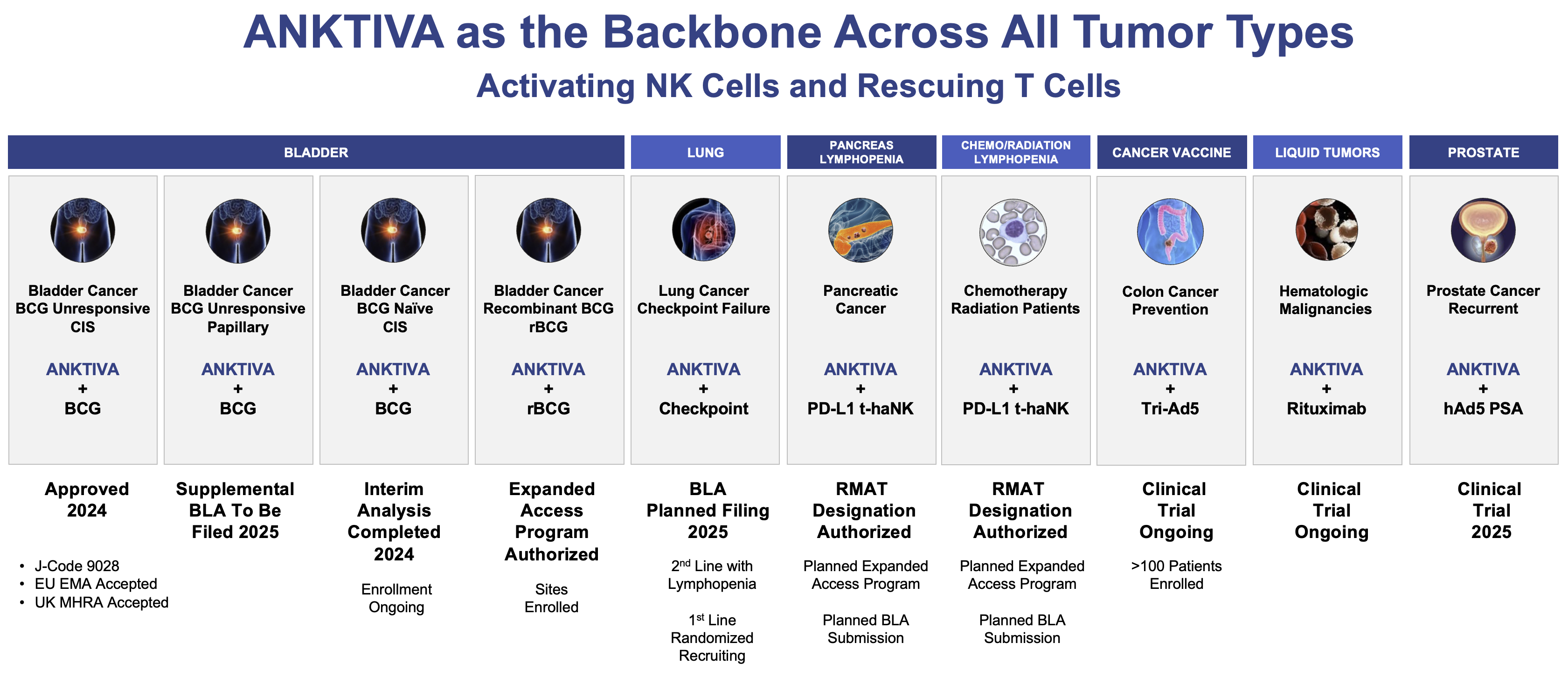
Select Clinical Development Program 2025+
The future of immunotherapy appears bright and I continue my quest to drive towards a therapeutic vaccine across multiple tumor types where patients do not suffer the adverse events of high-dose chemotherapy and high-dose radiotherapy. We have made and are targeting multiple submissions to the FDA in 2025 and beyond. In February2025, the FDA authorized an EAP allowing us to provide rBCG developed by Serum Institute to urologists to address the TICE BCG shortage in all settings where TICE BCG is approved.
In addition, as announced on February 27, 2025, the FDA has granted ImmunityBio RMAT designation for ANKTIVA and PD-L1 t-haNK in combination with standard-of-care chemotherapy/radiotherapy indicated for the reversal of lymphopenia and treatment of multiply relapsed locally advanced or metastatic pancreatic cancer. This RMAT designation follows clinical data of ALC and significant overall survival correlations in QUILT trials across multiple tumor types including third-line or greater metastatic pancreatic cancer, checkpoint relapsed NSCLC, and supportive data from healthy volunteers. The reversal of lymphopenia by ImmunityBio’s IL-15 receptor superagonist is consistent with the mechanism of action of ANKTIVA demonstrating proliferation and activation of NK cells, CD4+ T cells, CD8+ T cells, and memory T cells without upregulation of suppressive T reg cells and approved in the ANKTIVA label (approved for the treatment of adult patients with BCG-unresponsive NMIBC with CIS with or without papillary tumors). ImmunityBio intends to submit a BLA for the indication of reversal of lymphopenia in patients receiving standard-of-care chemotherapy and/or radiation and for the treatment of locally advanced or metastatic pancreatic cancer which includes the first-in-class CAR-NK (PD-L1 t-haNK), and to provide data from fully enrolled clinical trials in metastatic pancreatic cancer (QUILT 88) and in checkpoint relapsed NSCLC (QUILT 3055, NSCLC Cohort) patients, as well as lymphopenia reversal across multiple tumor types (QUILT 3055, all Cohorts), with supportive data of lymphocyte proliferation in healthy volunteers (QUILT 1004). In addition, ImmunityBio intends to file an EAP for ANKTIVA and PD-L1 t-haNK in combination with standard-of-care chemotherapy/radiotherapy and submit the protocol to the FDA.